Application of the aza-Diels–Alder reaction in the synthesis of natural products
Abstract
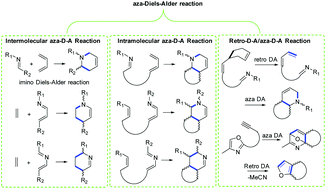
The Diels–Alder reaction that involves a nitrogen atom in the diene or dienophile is termed the aza-Diels–Alder reaction. As well as the powerful all-carbon Diels–Alder reaction, the aza-Diels–Alder reaction has also played an important role in the total synthesis of natural products. Herein, we review various natural products using an aza-Diels–Alder reaction as a key step to their total synthesis, and divide the syntheses into inter- and intra-molecular aza-Diels–Alder reactions and a retro-aza-Diels–Alder reaction. Inter- and intra-molecular aza-Diels–Alder reactions involve an imine as an electron deficient dienophile and an imine as an electron deficient azadiene. The significance of the aza-Diels–Alder reaction for the construction of a six-membered ring containing nitrogen is tremendous, but the development of asymmetric, in particular catalytic enantioselective intramolecular aza-Diels–Alder reaction in the total synthesis of natural products remains highly challenging, and will no doubt see enormous advances in the future.


 Please wait while we load your content...
Please wait while we load your content...