Divergent synthesis from reactions of 2-trifluoromethyl-1,3-conjugated enynes with N-acetylated 2-aminomalonates†
Abstract
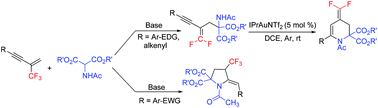
A simple base mediated reaction of 2-trifluoromethyl-1,3-conjugated enynes with N-acetylated 2-aminomalonates was developed, which could deliver two distinct types of products depending on substrates, i.e. 4-trifluoromethyl pyrrolidine derivatives, or gem-difluoro-1,3-conjugated enyne derivatives. Various functionalized 4-(difluoromethylene)-1,2,3,4-tetrahydropyridines could be obtained in good yields via the gold(I)-catalysed 6-endo-dig cyclization of the corresponding gem-difluoro-1,3-conjugated enynes under mild conditions.


 Please wait while we load your content...
Please wait while we load your content...