A dramatic synergistic effect of a flexible achiral linker on a rigid chiral cis-1,2-diamine bifunctional organocatalyst†
Abstract
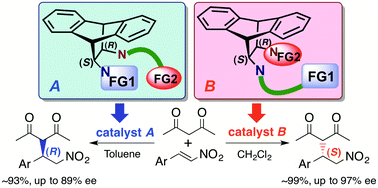
The combination of a “rigid” chiral bicyclic cis-1,2-diamine skeleton with steric bulkiness and a “flexible” achiral linker was newly designed as a bifunctional organocatalyst framework and it showed excellent catalytic activity of up to 0.05 mol%, accompanied by the reversal of enantioselection depending on the position of the linker, in an amine-thiourea organocatalyzed asymmetric Michael reaction.


 Please wait while we load your content...
Please wait while we load your content...