Synthetic water soluble di-/tritopic molecular receptors exhibiting Ca2+/Mg2+ exchange†
Abstract
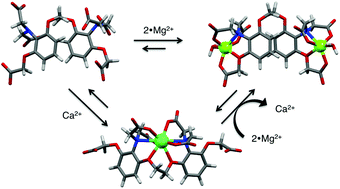
Structural integration of two synthetic water soluble receptors for Ca2+ and Mg2+, namely 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) and o-aminophenol-N,N,O-triacetic acid (APTRA), respectively, gave novel di- and tritopic ionophores (1 and 2). As Mg2+ and Ca2+ cannot be simultaneously complexed by the receptors, allosteric control of complexation results. Potentiometric measurements established stepwise protonation constants and showed high affinity for Ca2+ (log K = 6.08 and 8.70 for 1 and 2, respectively) and an excellent selectivity over Mg2+ (log K = 3.70 and 5.60 for 1 and 2, respectively), which is compatible with magnesium–calcium ion exchange. While ion-exchange of a single Mg2+ for a single Ca2+ is possible in both 1 and 2, the simultaneous binding of two Mg2+ by 2 appears prohibitive for replacement of these two ions by a single Ca2+. Ion-binding and exchange was further rationalized by DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...