Effect of solvent on radical cyclisation pathways: SRN1 vs. aryl–aryl bond forming mechanisms†
Abstract
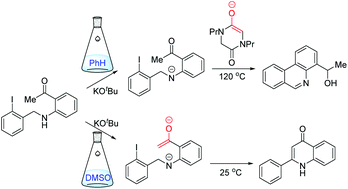
A recent paper identified a series of alternative cyclisation pathways of aryl radicals that resulted from electron transfer to various tethered haloarene–acetylarene substrates, in either benzene or DMSO as solvent. The electron transfer occurred from one of two enolates that were formed in the presence of KOtBu: either the enolate of the acetylarene, within the haloarene–acetylarene substrate, or the enolate 7 of the N,N′-dipropyl diketopiperazine (DKP) additive 6. This paper uses contemporary computational methods to determine the reaction pathways involved; depending on the substrate, the aryl radical underwent (i) SRN1 onto the enolate anion of the acetylarene, (ii) aryl–aryl bond formation, (iii) tandem hydrogen atom abstraction followed by SRN1 cyclisation and even (iv) ArC–N cleavage. The influence of the solvent was investigated. In this paper it is shown that the solvent influences which reactive species are present in the reaction mixture, and whether each species acts as an electron donor or an electron acceptor in the radical initiation or propagation steps. The main initiation step is a single electron transfer from the enolate anion 7 of the DKP additive in benzene, but in DMSO the initiation can occur from the enolate anion of the substrate itself. Using computational techniques a deeper understanding of the radical pathways involved has been obtained, which shows how we can use solvent to preferentially access products arising from either SRN1 or aryl–aryl bond formation pathways.


 Please wait while we load your content...
Please wait while we load your content...