Asymmetric synthesis of 4-aryl-1,2,5-thiadiazolidin-3-one 1,1-dioxides via Pd-catalyzed hydrogenation of cyclic ketimines†
Abstract
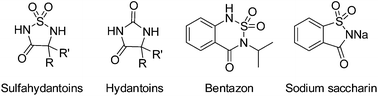
An efficient access to optically active sulfahydantoins, 4-aryl-1,2,5-thiadiazolidin-3-one 1,1-dioxides, was developed through palladium-catalyzed asymmetric hydrogenation of the corresponding cyclic N-sulfonylketimines with up to 98% ee.


 Please wait while we load your content...
Please wait while we load your content...