One-step synthesis of conjugated enynenitriles from bromocyanoacetylene†
Abstract
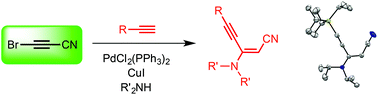
The chemical reactivity of bromocyanoacetylene has been evaluated for the first time by making it react with terminal alkynes and secondary amines in the presence of bis(triphenylphosphine)palladium dichloride and copper iodide as co-catalysts. This reaction provides new conjugated enynenitriles stereoselectively in one step in variable yields.


 Please wait while we load your content...
Please wait while we load your content...