An efficient Ugi-3CR/aza Diels–Alder/Pomeranz–Fritsch protocol towards novel aza-analogues of (±)-nuevamine, (±)-lennoxamine and magallanesine: a diversity oriented synthesis approach†
Abstract
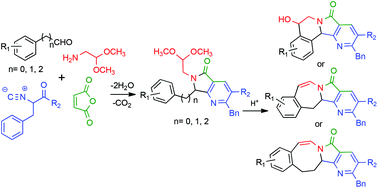
A rapid and efficient synthesis of a series of (±)-nuevamine, (±)-lennoxamine and magallanesine aza analogues is described. The synthetic strategy involves Ugi-3CR and two further condensation processes, aza-Diels–Alder cycloaddition and the Pomeranz–Fritsch reaction. The variation of the chain-size in aldehyde moieties provided structural diversity in only two operational reaction steps.


 Please wait while we load your content...
Please wait while we load your content...