A linear hydroxymethyl tetramate undergoes an acetylation–elimination process for exocyclic methylene formation in the biosynthetic pathway of pyrroindomycins†
Abstract
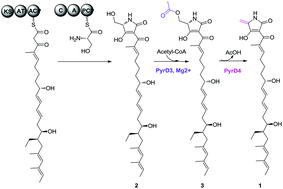
We herein report the isolation and characterization of a key linear intermediate in the biosynthetic pathway of pyrroindomycins, the potent spirotetramate natural products produced by Streptomyces rugosporus. This polyene intermediate bears a γ-hydroxymethyl group that is exocyclic to the tetramate moiety, indicating that a serine residue serves as the three-carbon unit for tetramate formation and chain-elongation termination. The further conversion involves an acetylation–elimination of the exocyclic γ-hydroxymethyl group to generate a γ-methylene group, which is indispensable for intramolecular [4 + 2] cross-bridging to construct the characteristic pentacyclic core. The findings presented in this study provide new insights into the biosynthesis of pyrroindomycins, and thus suggest a common paradigm for both spirotetramates and spirotetronates in processing the exocyclic γ-hydroxymethyl group of the five-membered heterocycle.


 Please wait while we load your content...
Please wait while we load your content...