Electronic halocyclization and radical haloazidation of benzene-linked 1,7-dienes for the synthesis of functionalized 3,1-benzoxazines†
Abstract
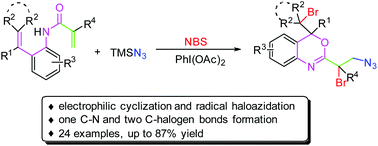
A novel electronic halocyclization and radical haloazidation of benzene-linked 1,7-dienes for the formation of functionalized 3,1-benzoxazines has been achieved by using TMSN3 as an azido source and NBS as a halogen source. This methodology is highlighted by its mild conditions and wide substrate scope, which concomitantly introduces one C–N and two C–halogen bonds into one molecule.

 Please wait while we load your content...
Please wait while we load your content...