Mechanistic investigations of the asymmetric hydrosilylation of ketimines with trichlorosilane reveals a dual activation model and an organocatalyst with enhanced efficiency†
Abstract
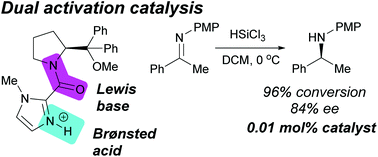
Structural probes used to help elucidate mechanistic information of the organocatalyzed asymmetric ketimine hydrosilylation have revealed a new catalyst with unprecedented catalytic activity, maintaining adequate performance at 0.01 mol% loading. A new ‘dual activation’ model has been proposed that relies on the presence of both a Lewis basic and Brønsted acidic site within the catalyst architecture.


 Please wait while we load your content...
Please wait while we load your content...