Oxidative decarbonylative coupling of aliphatic aldehydes with methacryloyl benzamides to generate isoquinoline-1,3(2H,4H)-diones†
Abstract
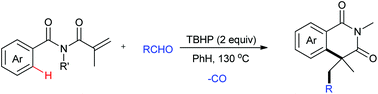
Alkyl-substituted isoquinoline-1,3(2H,4H)-diones were prepared by decarbonylative coupling of N-alkyl-N-methacryloylbenzamides and aliphatic aldehydes under metal-free conditions. The reaction undergoes subsequent decarbonylation, radical addition and cyclization processes with aliphatic aldehydes as the cheap and abundant alkyl radical source. This procedure offers a complementary approach to the convenient generation of alkyl-substituted isoquinoline-1,3(2H,4H)-diones.


 Please wait while we load your content...
Please wait while we load your content...