An efficient approach to trans-4-hydroxy-5-substituted 2-pyrrolidinones through a stereoselective tandem Barbier process: divergent syntheses of (3R,4S)-statines, (+)-preussin and (−)-hapalosin†
Abstract
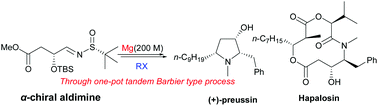
A diastereoselective approach to trans-4-hydroxy-5-substituted 2-pyrrolidinones 1 (P1 = TBS, P2 = H) has been developed through a stereoselective tandem Barbier process of (R,SRS)-8 with alkyl and aryl bromide. The stereochemistry at the C-5 stereogenic center of the trans-4-hydroxy-5-substituted 2-pyrrolidinones was solely controlled by α-alkoxy substitution. This effective approach was successfully used to prepare a variety of substituted (3R,4S)-statines 2. In addition, two bioactive natural products of (+)-preussin 4 and hapalosin 5 were effectively synthesized through this stereoselective tandem Barbier process.


 Please wait while we load your content...
Please wait while we load your content...