On the nature of the electronic effect of multiple hydroxyl groups in the 6-membered ring – the effects are additive but steric hindrance plays a role too†
Abstract
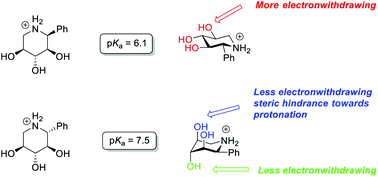
Research during the last two decades has shown a remarkable directional component of the substituent effects of hydroxy groups, which has a profound effect on the properties of hydroxylated compounds such as carbohydrates. While the epimerisation of a single hydroxyl function is well studied the consequence of multiple epimerisations is more speculative. In this work the effect of three epimerisations was investigated. To this end epimeric 2-phenyl iminoxylitols that have a phenyl group as a conformational anchor and thus hydroxyl groups in the axial or equatorial position, respectively, were synthesized and their pKa and conformation were studied. The results show that the large difference in the electronic effect between the axial and equatorial hydroxyls is partially cancelled by counteracting steric hindrance from 1,3-diaxial interactions. Hydrogen bonding does not appear to play any role in the electronic influence of the hydroxyl groups.


 Please wait while we load your content...
Please wait while we load your content...