A protecting-group-free synthesis of (+)-nephrosteranic, (+)-protolichesterinic, (+)-nephrosterinic, (+)-phaseolinic, (+)-rocellaric acids and (+)-methylenolactocin†
Abstract
A collective synthesis of a γ-butyrolactone class of paraconic acids such as (+)-methylenolactocin, (+)-phaseolinic acid, (+)-nephrosteranic acid, (+)-nephrosterinic acid, (+)-rocellaric acid and (+)-protolichesterinic acid is described. The strategy adopted is protecting-group-free based on efficient Pd-catalyzed Suzuki–Miyaura coupling and Ru-catalyzed Sharpless oxidation to construct the core β-CO2H-γ-butyrolactone unit to accomplish the synthesis of various paraconic acids.
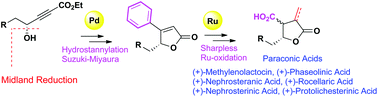


 Please wait while we load your content...
Please wait while we load your content...