Push–pull pyropheophorbides for nonlinear optical imaging†
Abstract
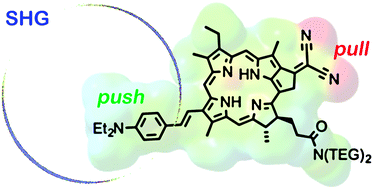
Pyropheophorbide-a methyl ester (PPa-OMe) has been modified by attaching electron-donor and -acceptor groups to alter its linear and nonlinear optical properties. Regioselective bromination of the terminal vinyl position and Suzuki coupling were used to attach a 4-(N,N-diethylaminophenyl) electron-donor group. The electron-acceptor dicyanomethylene was attached at the cyclic ketone position through a Knoevenagel condensation. Four different derivatives of PPa-OMe, containing either electron-donor or electron-acceptor groups, or both, were converted to hydrophilic bis-TEG amides to generate a series of amphiphilic dyes. The absorption and emission properties of all the dyes were compared to a previously reported push–pull type porphyrin-based dye and a commercial push–pull styryl dye, FM4-64. Electrochemical measurements reveal that the electron donor group causes a greater decrease in HOMO–LUMO gap than the electron-acceptor. TD-DFT calculations on optimized geometries (DFT) of all four dyes show that the HOMO is mostly localized on the donor, 4-(N,N-diethylaminophenyl), while the LUMO is distributed around the chlorin ring and the electron-acceptor. Hyper-Rayleigh scattering experiments show that the first-order hyperpolarizabilities of the dyes increase on attaching either electron-donor or -acceptor groups, having the highest value when both the donor and acceptor groups are attached. Two-photon excited fluorescence (TPEF) and second harmonic generation (SHG) images of the bis-TEG amide attached dyes in lipid monolayer-coated droplets of water-in-oil reveal that the TPEF and SHG involve transition dipole moments in different orientations.


 Please wait while we load your content...
Please wait while we load your content...