Second-generation total synthesis of aplyronine A featuring Ni/Cr-mediated coupling reactions†
Abstract
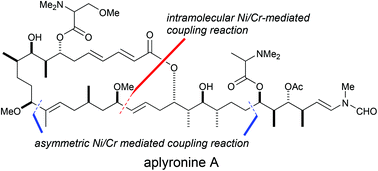
Second-generation total synthesis of aplyronine A, a potent antitumor marine macrolide, was achieved using Ni/Cr-mediated coupling reactions as key steps. The overall yield of the second-generation synthetic pathway of aplyronine A was 1.4%, obtained in 38 steps based on the longest linear sequence. Compared to our first-generation synthetic pathway of aplyronine A, the second-generation synthesis greatly improved both the yield and number of steps. In particular, we improved the stereoselectivity in the construction of the C13 stereogenic center and the C14–C15 (E)-trisubstituted double bond using the asymmetric Ni/Cr-mediated coupling reaction. Furthermore, we established efficient reaction conditions for the asymmetric Ni/Cr-mediated coupling reaction between the C21–C28 segment and C29–C34 segment. Thus, this coupling reaction proceeded with an equimolar ratio of each segment.

 Please wait while we load your content...
Please wait while we load your content...