A revised mechanism for the α-ketoacid hydroxylamine amide forming ligations†
Abstract
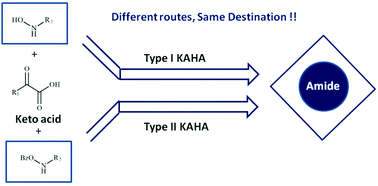
Computational investigations of the α-ketoacid-hydroxylamine amide-forming (KAHA) ligation of O-unsubstituted (type-I) and O-benzoyl substituted (type-II) hydroxylamine have revealed a distinct mechanistic pathway for the KAHA ligation reactions. Instead of a pathway involving lactone and oxiridine intermediates for the reaction of O-unsubstituted hydroxylamine and ketoacids (type-I KAHA), as had been proposed in the experimental studies, the computational results favor the pathway which involves migration of the hydroxy group (–OH) to the adjacent carbon in one of the key steps. The new pathway for the type I KAHA reaction explains the distribution of the 18O label in the final product (amide) that is observed in 18O labeling experiments of type-I ligation reaction. A coherent mechanistic course is also identified for the reaction of O-benzoyl substituted hydroxylamine and ketoacid (type II KAHA) reactions. The proposed pathway for the type-II KAHA ligation reaction proceeds with the retention of an oxygen atom of the keto group of ketoacids rather than hydroxylamine in the final product (amide). These findings are consistent with the results of 18O labeling experiments performed by Bode and coworkers on the KAHA reactions.

 Please wait while we load your content...
Please wait while we load your content...