Direct synthesis of γ-pyrones by electrophilic condensation of β-ketoesters†
Abstract
Triflic anhydride is a versatile electrophile that is able to activate poor nucleophiles. Herein, we show that readily available β-keto esters are activated by Tf2O furnishing γ-pyrones. Mechanistic studies suggest that this transformation proceeds via a double triflation, formation of an oxocarbenium intermediate and dealkylation promoted by a crucial nitrile additive.
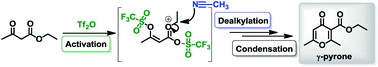

 Please wait while we load your content...
Please wait while we load your content...