Commensurability between protein nanotubes in contractile ejection nanomachines
Abstract
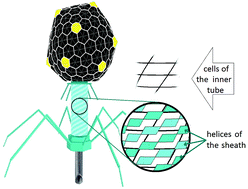
Contractile ejection nanomachines being sheath–tube assemblies create an opening in the cell membrane to translocate molecules or ions across it. Here, on the most structurally investigated examples of the bacteriophage T4 tail and pyocin R2, we show that the rearrangement of the sheath structure resulting in its contraction and twist occurs in such a way that the contracted sheath becomes commensurate with the inner tube. This fact dictates the previously unknown simple geometrical relationship between the nanotube symmetries. Using the Frank and van der Merwe classical theory of commensurability, we study an interaction between two protein nanotubes forming such nanomachines and obtain an expression for the corresponding energy, which depends on the tube structures and their mutual arrangement. The appearance of commensurability between the contracted sheath and the inner tube decreases both the interaction energy and the total energy of the system. It improves the nanomachine efficiency, since the energy gain obtained increases the torque of the inner tube piercing the cell membrane.


 Please wait while we load your content...
Please wait while we load your content...