Hierarchical cobalt–nitride and –oxide co-doped porous carbon nanostructures for highly efficient and durable bifunctional oxygen reaction electrocatalysts†
Abstract
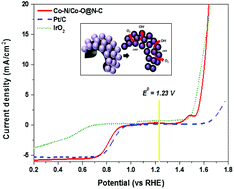
Here we report the preparation of hollow microspheres with a thin shell composed of mixed cobalt nitride (Co–N) and cobalt oxide (Co–O) nanofragments encapsulated in thin layers of nitrogen-doped carbon (N–C) nanostructure (Co–N/Co–O@N–C) arrays with enhanced bifunctional oxygen electrochemical performance. The hybrid structures are synthesized via heat treatment of N-doped hollow carbon microspheres with cobalt nitrate, and both the specific ratio of these precursors and the selected annealing temperature are found to be the key factors for the formation of the unique hybrid structure. The as-obtained product (Co–N/Co–O@N–C) presents a large specific surface area (493 m2 g−1), high-level heteroatom doping (Co–N, Co–O, and N–C), and hierarchical porous nanoarchitecture containing macroporous frameworks and mesoporous walls. Electronic interaction between the thin N–C layers and the encapsulated Co–N and Co–O nanofragments efficiently optimizes oxygen adsorption properties on the Co–N/Co–O@N–C and thereby triggers bifunctional oxygen electrochemical activity at the surface. The Co–N/Co–O@N–C nanohybrid exhibited a high onset potential of 0.93 V, and a limiting current density of 5.6 mA cm−2 indicating 4-electron oxygen reduction reaction (ORR), afforded high catalytic activity for the oxygen evolution reaction (OER) and even exceeded the catalytic stability of the commercial precious electrocatalysts; furthermore, when integrated into the oxygen electrode of a regenerative fuel cell device, it exhibited high-performance oxygen electrodes for both the ORR and the OER.


 Please wait while we load your content...
Please wait while we load your content...