Insights into the growth mechanism of REF3 (RE = La–Lu, Y) nanocrystals: hexagonal and/or orthorhombic†
Abstract
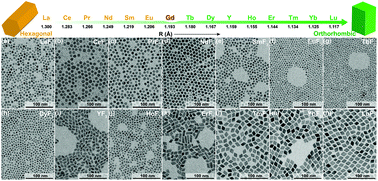
The synthesis of REF3 (RE = La–Lu, Y) nanocrystals with controlled phase structures has so far remained a challenge. Herein we have developed a one-for-all synthetic procedure that allows the successful synthesis of REF3 nanocrystals in a controlled manner. Experimental results showed that the radius of RE ions determines the phase structure: pure hexagonal REF3 (RE = La–Eu), a mixture of hexagonal and orthorhombic REF3 (RE = Gd), and pure orthorhombic REF3 (RE = Tb–Lu, Y) nanocrystals are obtained along with the decrease of the ionic radius. As Gd is positioned exactly in the middle of the lanthanides row, GdF3 nanocrystals were used as a model to further investigate how the molar ratio of F− : Gd3+, the doping of RE ions with different ionic radii, and the doping concentration of certain RE ions affects the crystal structure of the final product.


 Please wait while we load your content...
Please wait while we load your content...