Evaluation of mesoporous silica nanoparticles for oral drug delivery – current status and perspective of MSNs drug carriers
Abstract
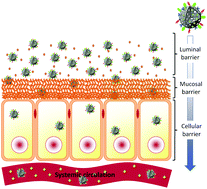
The oral pathway is considered as the most common method for drug administration, although many drugs, especially the highly pH- and/or enzymatic biodegradable peptide drugs, are very difficult to formulate and achieve a good intestinal absorption. Efficient systematic absorption of an active substance, delivered via oral ingestion, is only achievable if the drug (1) is substantially present as a solution in the gastrointestinal tract, (2) is able to penetrate through the intestinal mucus, (3) overcomes the different gastrointestinal barriers, and (4) provides an effective therapeutic dose. Therefore, optimization of oral bioavailability of poorly-soluble drugs still remains a significant challenge for the pharmaceutical industry. Even though numerous conventional drug carriers have successfully solved some of the issues related to oral delivery of poorly-soluble drugs, only few of them met commercialization requirements. These drawbacks have led the scientific world to reconsider its approaches toward targeted drug delivery systems and researchers started looking for alternative vectorized carriers. In this area, nanoparticle-based materials have several significant advantages over free and non-formulated drugs. For example, nanosized porous silica carriers allow for more sustained and controlled drug release or improved oral bioavailability. Thus, in the present review, we will highlight the most important features of nanostructured silica drug carriers, such as particle size, particle shape, surface roughness or surface functionalization, and underline the key advantages of these nanosupports. In particular, this article will discuss recent progress and challenges in the area of mesoporous silica nanocarriers used for oral drug delivery. Additional emphasis will be set on the biological and chemical features of the gastrointestinal tract as well as currently tested nanoformulations and strategies to avoid drug degradation in the gastrointestinal environment.
- This article is part of the themed collection: Recent Review Articles


 Please wait while we load your content...
Please wait while we load your content...