Capabilities of transition metals in retarding the bonding of carbon atoms to minimize dendritic graphene†
Abstract
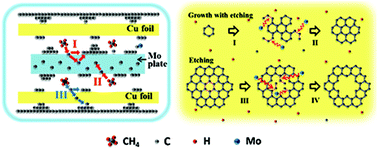
The avoidance of growing dendritic graphene on the copper substrate during the chemical vapor deposition process is greatly desired. Here we have identified a mechanism, in which (1) transition metal plates placed inside the copper pockets reduce the majority of active carbon atoms to eventually suppress the graphene growth rate, and (2) transition metals etch graphene C–C bonds along defective edges to grow into zigzag-edge ending domains with higher priorities. Via isotopic labeling of the methane method, we have observed bright-dark-alternating hexagonal-shaped rings, which are shown in Raman mapping images. Under a hydrogen atmosphere, we are capable of acquiring hexagonal openings within graphene domains by means of transition-metal-driven catalytic etching. This methodology may work as a simple and convenient way to determine graphene size and crystal orientation, and may enable the etching of graphene into smooth and ordered zigzag edge nanoribbons without compromising the quality of graphene.


 Please wait while we load your content...
Please wait while we load your content...