Mitochondrial-targeted multifunctional mesoporous Au@Pt nanoparticles for dual-mode photodynamic and photothermal therapy of cancers†
Abstract
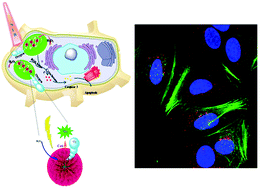
In the conventional non-invasive cancer treatments, such as photodynamic therapy (PDT) and photothermal therapy (PTT), light irradiation is precisely focused on tumors to induce apoptosis via the generation of reactive oxygen species (ROS) or localized heating. However, overconsumption of oxygen and restricted diffusion distance of ROS limit the therapeutic effects on hypoxic tumors. Herein, we developed a platform for the rapid uptake of multifunctionalized Au@Pt nanoparticles (NPs) by mitochondria in cancer cells. The mesoporous Au@Pt nanoparticles were labeled with a cell-targeting ligand (folic acid), a mitochondria-targeting group (triphenylphosphine (TPP)), and a photosensitizer (Ce6). This led to significant improvement of the PDT efficacy due to an enhanced cellular uptake, an effective mitochondrial ROS burst, and a rapid intelligent release of oxygen. Moreover, Au@Pt NPs can convert laser radiation into heat, resulting in thermally induced cell damage. This nanosystem could be used as a dual-mode phototherapeutic agent for enhanced cancer therapy and molecular targets associated with disease progression. We achieved a mitochondria-targeted multifunctional therapy strategy (a combination of PDT and PTT) to substantially improve the therapeutic efficiency.
- This article is part of the themed collection: 2017 Nanoscale HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...