Morphometric characterization of fibrinogen's αC regions and their role in fibrin self-assembly and molecular organization†
Abstract
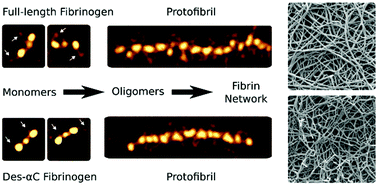
The flexible C-terminal parts of fibrinogen's Aα chains named the αC regions have been shown to play a role in fibrin self-assembly, although many aspects of their structure and functions remain unknown. To examine the involvement of the αC regions in the early stages of fibrin formation, we used high-resolution atomic force microscopy to image fibrinogen and oligomeric fibrin. Plasma-purified full-length human fibrinogen or des-αC fibrinogen lacking most of the αC regions, untreated or treated with thrombin, was imaged. Up to 80% of the potentially existing αC regions were visualized and quantified; they were highly heterogeneous in their length and configurations. Conversion of fibrinogen to fibrin was accompanied by an increase in the incidence and length of the αC regions as well as transitions from more compact conformations, such as a globule on a string, to extended and more flexible offshoots. Concurrent dynamic turbidimetry, confocal microscopy, and scanning electron microscopy revealed that trimming of the αC regions slowed down fibrin formation, which correlated with longer protofibrils, thinner fibers, and a denser network. No structural distinctions, except for the incidence of the αC regions, were revealed in the laterally aggregated protofibrils made of the full-length or des-αC fibrinogens, suggesting a pure kinetic effect of the αC regions on the fibrin architecture. This work provides a structural molecular basis for the promoting role of the αC regions in the early stages of fibrin self-assembly and reveals this stage of fibrin formation as a potential therapeutic target to modulate the structure and mechanical properties of blood clots.


 Please wait while we load your content...
Please wait while we load your content...