Enzyme-assisted peptide folding, assembly and anti-cancer properties†
Abstract
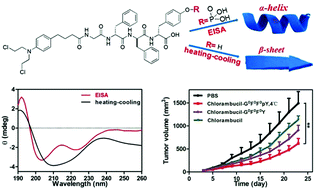
The α-helix is the most prevalent conformation in proteins. However, formation of the α-helical conformation remains a challenge for short peptides with less than 5 amino acids. We demonstrated in this study that enzyme-instructed self-assembly (EISA) provides a unique pathway to assist the self-assembly of peptides into the α-helical conformation, while a heating–cooling process leads to a conformation more similar to a β-sheet. The same peptide with different conformations self-assembled into different nanostructures. The peptide with α-helical conformation self-assembled into stable nanofibers and hydrogels, while the other one assembled into an unstable nanoparticle suspension. The nanofiber solution exhibited better stability against proteinase K digestion and an enhanced cellular uptake compared to the nanoparticle solution. Therefore, the nanomedicine formed by the α-helical peptide showed a better inhibition capacity against cancer cells in vitro and significantly inhibited tumor growth in vivo compared to the one formed by the β-sheet peptide. Our study demonstrates the unique advantages of EISA to assist peptide folding and self-assembly into biofunctional nanomaterials.


 Please wait while we load your content...
Please wait while we load your content...