Anion-exchange synthesis of a nanoporous crystalline CoB2O4 nanowire array for high-performance water oxidation electrocatalysis in borate solution†
Abstract
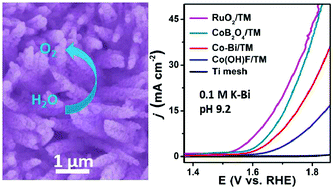
Developing nanoporous nanoarray electrocatalysts for efficient water oxidation in environmentally benign media is highly desired but still remains a key challenge. In this communication, we report the fabrication of a nanoporous crystalline CoB2O4 nanowire array on Ti mesh (CoB2O4/TM) from a Co(OH)F nanowire array on Ti mesh (Co(OH)F/TM) via an anion-exchange reaction. As a three dimensional (3D) catalyst electrode for water oxidation, CoB2O4/TM exhibits superior catalytic activity and needs an overpotential of only 446 mV to drive a geometrical catalytic current density of 10 mA cm−2 in 0.1 M potassium borate (pH = 9.2). Notably, this catalyst also shows strong long-term electrochemical durability with high turnover frequency values of 0.19 and 0.81 mol O2 per s at overpotentials of 400 and 500 mV, respectively.


 Please wait while we load your content...
Please wait while we load your content...