Controlled electrochemical doping of graphene-based 3D nanoarchitecture electrodes for supercapacitors and capacitive deionisation†
Abstract
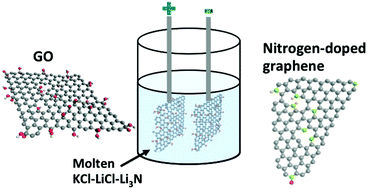
Chemically-doped graphenes are promising electrode materials for energy storage and electrosorption applications. Here, an affordable electrochemical green process is introduced to dope graphene with nitrogen. The process is based on reversing the polarity of two identical graphene oxide (GO) electrodes in molten KCl–LiCl–Li3N. During the cathodic step, the oxygen functional groups on the GO surface are removed through direct electro-deoxidation reactions or a reaction with the deposited lithium. In the anodic step, nitrogen is adsorbed onto the surface of graphene and subsequently reacts to form nitrogen-doped graphene. The doping process is controllable, and graphene with up to 7.4 at% nitrogen can be produced. The electrochemically treated electrodes show a specific capacitance of 320 F g−1 in an aqueous KOH electrolyte and maintain 96% of this value after 10 000 cycles. The electrodes also display excellent electrosorption performance in capacitive deionisation devices with the salt removal efficiency reaching up to 18.6 mg g−1.


 Please wait while we load your content...
Please wait while we load your content...