Static and dynamic hidden symmetries of icosahedral viral capsids
Abstract
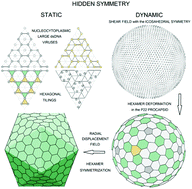
Viral shells self-assemble from identical proteins, which tend to form equivalent environments in the resulting assembly. However, in icosahedral capsids containing more than 60 proteins, they are enforced to occupy not only the symmetrically equivalent locations but also the quasi-equivalent ones. Due to this important fact, static and dynamic symmetries of viral shells can include additional hidden components. Here, developing the Caspar and Klug ideas concerning the quasi-equivalence of protein environments, we derive the simplest hexagonal tilings, that in principle could correspond to the local protein order in viral shells, and apply the resulting theory to nucleocytoplasmic large dsDNA viruses. In addition, analyzing the dynamic symmetry of the P22 viral shell, we demonstrate that the collective critical modes responsible for the protein reorganization during the procapsid maturation are approximately equivalent to the normal modes of the isotropic spherical membrane with O(3) symmetry. Furthermore, we establish the relationship between the dynamic symmetry of the P22 procapsid and the protein arrangement regularities that appear only in the mature capsid.


 Please wait while we load your content...
Please wait while we load your content...