Red-emitted electrochemiluminescence by yellow fluorescent thioglycol/glutathione dual thiolate co-coated Au nanoclusters†
Abstract
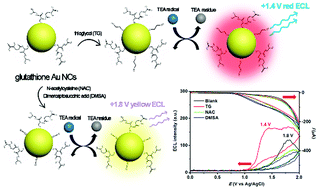
This study reports the occurrence of a special red-emitted anodic electrochemiluminescence (ECL) emission at +1.4 V (vs. Ag/AgCl) on a glass carbon electrode (GCE) after the addition of thioglycol (TG) to surface-unsaturated glutathione (GSH)-coated Au nanoclusters (NCs), with an emission peak at ∼630 nm. Compared to the ECL at a potential of +1.8 V (vs. Ag/AgCl) and an emission peak at 580 nm (corresponding to fluorescence) for only GSH-coated Au NCs, this ECL emission not only exhibits a lower ECL potential but also shows a significantly red-shifted emission wavelength up to ∼50 nm. We demonstrated that the formation of TG/GSH dual ligand-coated Au NCs is responsible for the red-shifted ECL emission. Other common thiol compounds cannot result in similar effect on GCE, and no ECL is observed on other electrodes such as indium tin oxide and platinum electrodes. This finding offers a great possibility to design novel feasible ECL systems for different complicated applications.


 Please wait while we load your content...
Please wait while we load your content...