Halogenation dictates the architecture of amyloid peptide nanostructures†
Abstract
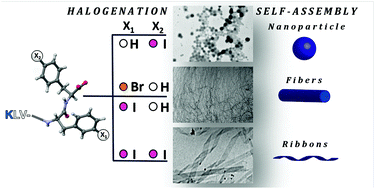
Amyloid peptides yield a plethora of interesting nanostructures though difficult to control. Here we report that depending on the number, position, and nature of the halogen atoms introduced into either one or both phenylalanine benzene rings of the amyloid β peptide-derived core-sequence KLVFF, four different architectures were obtained in a controlled manner. Our findings demonstrate that halogenation may develop as a general strategy to engineer amyloidal peptide self-assembly and obtain new amyloidal nanostructures.


 Please wait while we load your content...
Please wait while we load your content...