Why nanoscale tank treads move? Structures, chemical bonding, and molecular dynamics of a doped boron cluster B10C†
Abstract
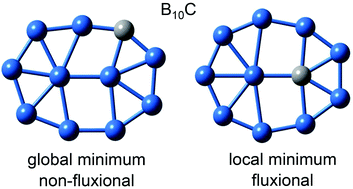
Planar boron clusters form dynamic rotors, either as molecular Wankel motors or subnanoscale tank treads, the latter being exemplified by an elongated B11− cluster. For an in-depth mechanistic understanding of the rotors, we investigate herein a doped boron cluster, B10C, in which a C atom isovalently substitutes B− in the B11− tank tread. Two critical structures are achieved: the Cs (1A′) global minimum (GM) with C positioned in the peripheral ring and the C2v (1A1) local minimum (LM) with C in the diatomic core. In the GM the C atom completely halts the rotation of B10C, whereas in the LM the dynamic fluxionality remains. The energy barriers for in-plane rotation differ markedly: 12.93/18.31 kcal mol−1 for GM versus 1.84 kcal mol−1 for LM at the single-point CCSD(T) level. The GM rotates via two transition states (TS), compared to one for the LM. Chemical bonding in the structures is elucidated via canonical molecular orbital (CMO) analysis, adaptive natural density partitioning (AdNDP), electron localization functions (ELFs), and Wiberg bond indices (WBI). Electron delocalization is shown to be essential for structural fluxionality. In particular, the variation of WBI from the GM or LM geometries to their TS structures correlates positively with the energy barrier, which offers a quasi-quantitative measure of the barrier height and hence controls the dynamics. This finding may be extended to all molecular rotors. It also helps rationalize why a strongly covalently bound system can behave dynamically in a manner similar to a weakly bound one; it is the latter that is generally anticipated to be structurally fluxional.


 Please wait while we load your content...
Please wait while we load your content...