Quintuple super bonding between the superatoms of metallic clusters†
Abstract
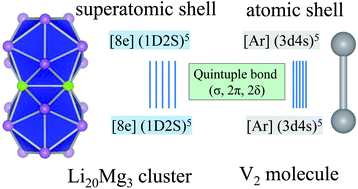
The synthesis of a stable compound with Cr–Cr quintuple bonding (σ, 2π, 2δ) opened the door to a new field of chemistry (T. Nguyen, A. D. Sutton, M. Brynda, J. C. Fettinger, G. J. Long and P. P. Power, Science, 2005, 310, 844). Looking back to the mass experiments on sodium clusters (W. D. Knight, K. Clemenger, W. A. de Heer, W. A. Saunders, M. Y. Chou and M. L. Cohen, Phys. Rev. Lett., 1984, 52, 2141), this work tells some new stories about the experimentally viewed magic numbers 26e and 30e. By unbiased global search, the 26e Li20Mg3 cluster has a perfect double-icosahedral motif with a large HOMO–LUMO energy gap (1.44 eV). We theoretically found that each icosahedron is an independent superatom and molecule-like electronic shell-closure is achieved via quintuple super bonding between two superatoms: [8e](1D2S)5–(1D2S)5[8e]. Similar quintuple bonding also exists in the 30e double-icosahedral Li18Mg3Al2 cluster: [8e](1D2S)7–(1D2S)7[8e]. The 26e/30e quintuple bonding was verified by the beautiful analogies in molecular orbital diagrams and chemical bonding patterns with V2/Re2 molecules. Such a quintuple super bonding makes a bridge between the jellium model and chemical bonding, which further expands the community of chemical bonds.


 Please wait while we load your content...
Please wait while we load your content...