The role of metal oxide interactions: revisiting Pt growth on the TiO2 surface in the process of impregnation method†
Abstract
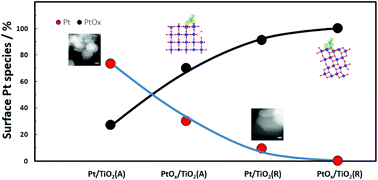
First-principles calculations and experiments with PtOx on TiO2 surfaces were performed together to understand the interactions of metal oxides during the calcination process and their influence on the growth pattern of Pt on the TiO2 surface. Our calculations indicate that PtOx with a high concentration of oxygen binds more strongly to rutile than to anatase, indicating the formation of stronger interactions between Pt oxide and the rutile surface compared with anatase during the calcination stage under an oxidative atmosphere. X-ray photoelectron spectra quantification analysis illustrates that higher amounts of Pt oxide are obtained when impregnation is performed with a rutile support after calcination in comparison with that of anatase. After reduction, the stronger interaction between PtOx and rutile leads to a larger amount of partially charged and highly dispersed Pt nanoclusters on the surface, while the weaker interaction between PtOx and anatase illustrates the dispersion and sintering of higher amounts of metal nanoparticles on the anatase surface. Furthermore, the photocatalytic oxygen evolution test highlights the importance of understanding the interaction between the metal oxide and anatase/rutile for targeted synthesis of the supported catalyst.


 Please wait while we load your content...
Please wait while we load your content...