The transition metal surface dependent methane decomposition in graphene chemical vapor deposition growth†
Abstract
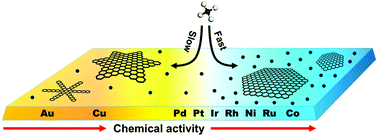
By using density-functional theory (DFT) calculations, the dissociation of CH4 on various metal surfaces, including Ni, Cu, Ru, Pd, Pt, Ir, Co, Au, and Rh, is systematically explored. For all the explored face-centered cubic (fcc) metal substrates, the (100) surface is found to be more active than the (111) surface, which explains the higher activity of the (100) surface in graphene chemical vapor deposition (CVD) growth. The catalytic activity order of these metals is found to be Ni ≈ Rh ≈ Co ≈ Ru > Pd ≈ Pt ≈ Ir > Cu > Au, which explained the catalyst type dependent growth behavior of graphene. It was found that the main dissociation product of CH4 on Ni, Pd, Pt, Ir, Rh, Co, and Ru substrates is a carbon monomer and a very high rate of dissociation is expected, but a low rate of dissociation and the dissociation products of CHi (i = 1, 2, 3) are expected on Cu and Au surfaces, which explained the diffusion-limited growth of graphene on Cu and Au surfaces and attachment limited growth on other active metal surfaces. Furthermore, our study shows that the dissociation of CH4 on all these metal substrates follows the well-known Brønsted–Evans–Polanyi (BEP) principles, or the reaction barrier is roughly linear to the reaction energy.


 Please wait while we load your content...
Please wait while we load your content...