Functional single-walled carbon nanotubes ‘CAR’ for targeting dopamine delivery into the brain of parkinsonian mice†
Abstract
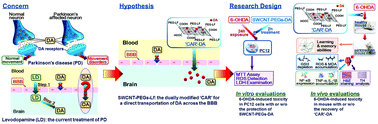
Current treatments for Parkinson's disease (PD) are limited, partly due to the difficulties posed by the blood brain barrier (BBB) when delivering drugs to the brain. Herein, we explore the feasibility and efficacy of functional single-walled carbon nanotubes ‘CAR’ (SWCNT-PEGs-Lf) which carry and target-deliver dopamine (DA) to the brain in PD mice for treatment. SWCNTs can penetrate the cell-membrane remarkably, with the characteristics including high drug-loading and pH-dependent therapeutic unloading capacities. It has been reported that polyethylene glycol (PEG)-coated SWCNTs could increase the circulation time and thus prolong the concentration gradient of SWCNTs to the brain. Besides, an obvious lactoferrin-nanoparticle (Lf-NP) accumulation in the striatum, wherein the pharmacological target site of PD has been reported, a dual modification of PEG and Lf onto SWCNTs was applied and thus a specific ‘CAR’ to carry DA. The results from in vitro studies demonstrate that with 20 mol L−1 DA loaded onto SWCNT-polyethylene glycol (PEGs) in addition to 100 μmol L−1 6-hydroxydopamine (6-OHDA), the activity of PC12 cells increases significantly (p < 0.05), and that the lactate dehydrogenase (LDH) levels and reactive oxygen species (ROS) content also significantly decrease (p < 0.01). Furthermore, the levels of oxidative stress, tumor necrosis factor (TNF)-α and interleukin (IL)-1β are all reduced significantly in PD mice and the CAR-25 mg kg−1 DA group in comparison with that in 6-OHDA-lesioned mice with saline and 6-OHDA-lesioned mice, as well as the Tyrosine hydroxylase-immunoreactive (TH-ir) density increased (p < 0.01). The toxicity of CAR was in vitro and in vivo investigated, showing that the safe dose of SWCNT-PEG exposure to PC12 cells was 6.25 μg μl−1 or lower with a higher metabolic activity in comparison with that in the control group and the safe dose of CAR in the mice experiments was 3.25 mg kg−1 or less, given by intraperitoneal injection with a lower level of oxidative stress and inflammatory responses in comparison with that in the control group. This study suggests that 25 mg kg−1 DA loaded onto 3.25 mg kg−1 CAR can alleviate the oxidative stress and inflammatory responses in parkinsonian mice and increase the TH-ir density in the striatum.


 Please wait while we load your content...
Please wait while we load your content...