The effect of surface ligands on the optical activity of mercury sulfide nanoparticles†
Abstract
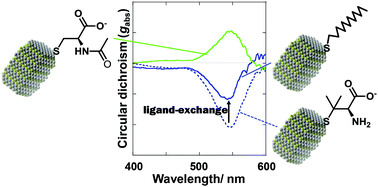
Mercury sulfide (HgS) nanoparticles (NPs) were prepared in the presence of water-soluble thiols as capping ligands in aqueous solutions. Chiral thiol ligands successfully afforded the formation of the chiral cinnabar phase (α-HgS), leading to optically active NPs, while two achiral thiols preserved β-HgS NPs with an achiral crystalline system. The profiles of UV-vis absorption and circular dichroism (CD) spectra of chiral NPs were dependent on the chemical structures of the chiral ligands. Cysteine-based derivatives gave HgS NPs demonstrating almost mirror image CD profiles even though they possess identical stereochemistry. The water soluble chiral ligands on the NPs were replaced with an achiral ligand, 1-dodecanethiol, by the spontaneous phase transfer method. The ligand-exchanged NPs with the achiral thiol preserved the optical activity with a feature of the CD profile similar to that of the original NPs in water, demonstrating the chiral memory effect in the NP-core. The dissymmetry factor in optical absorption decreased by almost half, which could be attributed to the amorphous phase formed by the chemical etching with an excess amount of dodecanethiol. The optical activity showed a higher thermal stability compared to that of NPs before the ligand-exchange.


 Please wait while we load your content...
Please wait while we load your content...