Charge storage performances and mechanisms of MnO2 nanospheres, nanorods, nanotubes and nanosheets †
Abstract
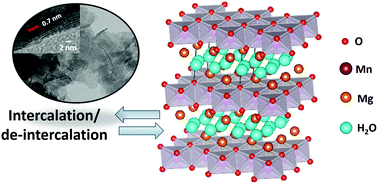
Manganese dioxide (MnO2) has been widely used as an active material for high-performance supercapacitors due to its high theoretical capacitance, high cycling stability, low cost, and environmental friendliness. However, the effect of its crystallographic phase on charge storage performances and mechanisms is not yet clear. Herein, MnO2-based supercapacitors with different structures including nanospheres, nanorods, nanotubes, and nanosheets have been fabricated and investigated. Among such structures, δ-MnO2 nanosheets exhibit the highest specific capacitance of 194.3 F g−1 at 1 A g−1 when compared with other phases and shapes. The maximum specific energy of the δ-MnO2 nanosheet supercapacitor is 23.4 W h kg−1 at 971.6 W kg−1 and the maximum specific power is 4009.2 W kg−1 at 15.9 W h kg−1 with a capacity retention of 97% over 15 000 cycles. The δ-MnO2 nanosheet mainly stores charges via a diffusion-controlled mechanism at the scan rates of 10–100 mV s−1, whilst the α-MnO2 with different morphologies including nanospheres, nanorods, and nanotubes store charges via a non-faradaic or non-diffusion controlled process especially at fast scan rates (50–100 mV s−1). Understanding the charge storage performance and mechanism of the MnO2 nanostructures with different crystallographic phases and morphologies may lead to the further development of supercapacitors.


 Please wait while we load your content...
Please wait while we load your content...