Confining the spin between two metal atoms within the carbon cage: redox-active metal–metal bonds in dimetallofullerenes and their stable cation radicals†
Abstract
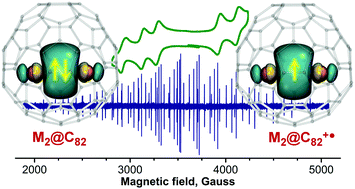
Lanthanide–lanthanide bonds are exceptionally rare, and dimetallofullerenes provide a unique possibility to stabilize and study these unusual bonding patterns. The presence of metal–metal bonds and consequences thereof for the electronic properties of M2@C82 (M = Sc, Er, Lu) are addressed by electrochemistry, electron paramagnetic resonance, SQUID magnetometry and other spectroscopic techniques. A simplified non-chromatographic separation procedure is developed for the isolation of Er2@C82 (Cs(6) and C3v(8) cage isomers) and Sc2@C82 (C3v(8) isomer) from fullerene mixtures. Sulfide clusterfullerenes Er2S@C82 with Cs(6) and C3v(8) fullerene cages are synthesized for the first time. The metal–metal bonding orbital of the spd hybrid character in M2@C82 is shown to be the highest occupied molecular orbital, which undergoes reversible single-electron oxidation with a metal-dependent oxidation potential. Sulfide clusterfullerenes with a fullerene-based HOMO have more positive oxidation potentials. The metal-based oxidation of Sc2@C82-C3v is confirmed by the EPR spectrum of the cation radical [Sc2@C82-C3v]+ generated by chemical oxidation in solution. The spectrum exhibits an exceptionally large a(45Sc) hyperfine coupling constant of 199.2 G, indicating a substantial 4s contribution to the metal–metal bonding orbital. The cationic salt [Er2@C82-C3v]+SbCl6− is prepared, and its magnetization behavior is compared to that of pristine Er2@C82-C3v and Er2S@C82-C3v. The formation of the single-electron Er–Er bond in the cation dramatically changes the coupling between magnetic moments of Er ions.


 Please wait while we load your content...
Please wait while we load your content...