A dual-ion electrochemistry deionization system based on AgCl-Na0.44MnO2 electrodes†
Abstract
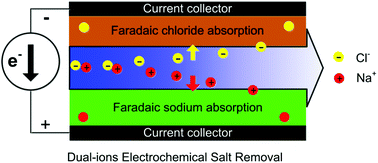
Novel desalination technologies with high ion removal capacity and low energy consumption are desirable to tackle the water shortage challenge. Herein, we report a dual-ion electrochemistry deionization (DEDI) system with silver chloride as the electrochemical chloride release/capture anode, sodium manganese oxide as the electrochemical sodium release/capture cathode, and flow salt solution as the electrolyte. Sodium and chloride ions are synergistically released to the flow electrolyte feed at an applied positive current. Under negative current conditions, the two ions are extracted from the flow electrolyte feed to their corresponding electrodes at the same time, which can cause a conductivity decrease indicating salt removal. The salt absorption/desorption capacity of the novel deionization system is stable and reversible, up to 57.4 mg g−1 for 100 cycles, which is much higher than that obtained by conventional or hybrid capacitive deionization devices. The charge efficiency is 0.979/0.956 during the salt desorption/absorption process. This research will be of great significance for high efficiency and low energy consumption seawater desalination.


 Please wait while we load your content...
Please wait while we load your content...