Heterogeneous Cu–Pd binary interface boosts stability and mass activity of atomic Pt clusters in the oxygen reduction reaction†
Abstract
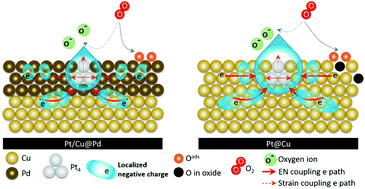
A ternary metallic CuPdPt nanocatalyst (NC) is synthesized using a wet chemical reduction method, which is sequentially designed, in the presence of acid treated carbon nanotubes. This NC is a nanocrystal with a configuration of a Cu@Pd core and atomic Pt clusters (∼9 wt%) on the top (Cu@Pd/Pt). A residual current of 92.6%, 5.2 times higher than that of commercial Pt catalysts (at 0.85 V vs. RHE), is retained after 40 000 cycles of an accelerated degradation test (ADT). Atomic and electronic structure analyses show that such exclusive stability mainly results from electron localization at Pt clusters in heterogeneous interfaces of the Cu–Pd core. Most importantly, we develop a robust ternary NC, which shows outstanding MA, superior chemical durability, and ∼90 wt% lower Pt loading than commercial Pt NCs in the oxygen reduction reaction.


 Please wait while we load your content...
Please wait while we load your content...