Insight into the hydrogen evolution reaction of nickel dichalcogenide nanosheets: activities related to non-metal ligands†
Abstract
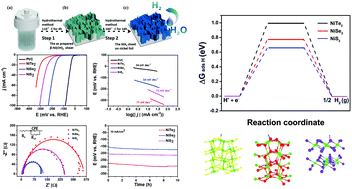
Transition metal dichalcogenides, MX2 (M = Fe, Co, Ni, X = S, Se, Te), have been proven to be promising substitutes for noble metals in hydrogen evolution reactions (HERs). However, forthright comparisons of metal sulfides, metal selenides, and metal tellurides are rarely conducted, let alone the mechanism of the important role of their non-metal ligands. In this paper, we report the pilot study of a controllable method for the preparation of a series of NiX2 (X = S, Se, Te) nanosheets via a facile anion-exchange reaction. Consequently, the HER activities and stabilities of NiS2, NiSe2, and NiTe2 nanosheets were tested in both acid and alkaline solutions. The required overpotentials to reach 10 mA cm−2 in 0.5 M H2SO4 for NiS2, NiSe2, and NiTe2 were 213, 156, and 276 mV, respectively. The best performance of NiSe2 was also confirmed in 1 M KOH. Besides NiS2 and NiTe2 nanosheets, the HER properties of NiSe2 nanosheets are superior to most of the available nickel catalysts. Interestingly, the results from electrochemical measurements were found to be fully consistent with the data based on density function theory calculation. Among various factors that might influence the HER activities of nickel dichalcogenides, the free energies of hydrogen adsorption and conductivities have played important roles.


 Please wait while we load your content...
Please wait while we load your content...