Proline-rich antimicrobial peptides targeting protein synthesis
Abstract
Covering: up to 2017
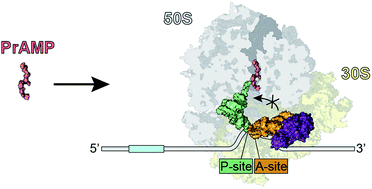
The innate immune system employs a broad array of antimicrobial peptides (AMPs) to attack invading microorganisms. While most AMPs act by permeabilizing the bacterial membrane, specific subclasses of AMPs have been identified that pass through membranes and inhibit bacterial growth by targeting fundamental intracellular processes. One such subclass is the proline-rich antimicrobial peptides (PrAMPs) that bind to the ribosome and interfere with the process of protein synthesis. A diverse range of PrAMPs have been identified in insects, such as bees, wasps and beetles, and crustaceans, such as crabs, as well as in mammals, such as cows, sheep, goats and pigs. Mechanistically, the best-characterized PrAMPs are the insect oncocins, such as Onc112, and bovine bactenecins, such as Bac7. Biochemical and structural studies have revealed that these PrAMPs bind within the ribosomal exit tunnel with a reverse orientation compared to a nascent polypeptide chain. The PrAMPs allow initiation but prevent the transition into the elongation phase of translation. Insight into the interactions of PrAMPs with their ribosomal target provides the opportunity to further develop these peptides as novel antimicrobial agents.
- This article is part of the themed collection: Novel Antibiotics and Antimicrobial Resistance


 Please wait while we load your content...
Please wait while we load your content...