Conformational control of the bacterial Clp protease by natural product antibiotics
Abstract
Covering: up to 2017
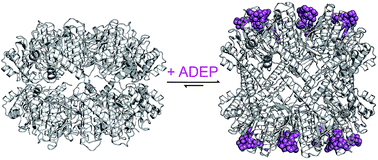
The bacterial Clp protease is a highly conserved and structurally versatile machine. It has gained a lot of recognition during the last decade as a novel antibacterial drug target with an unprecedented mechanism of action. Due to its complexity, there are distinct means of interfering with its natural functions and several compounds targeting this machine have been identified. In this review, we summarize the current state of knowledge about natural products deregulating Clp proteolysis, a crucial and delicate process within the cell. Among those, acyldepsipeptide antibiotics of the ADEP class (ADEPs) are characterized best. The molecular mechanism of ADEP-mediated deregulation sheds light on the inner workings of the Clp protease.
- This article is part of the themed collections: Celebrating Excellence in Research: 100 Women of Chemistry and Novel Antibiotics and Antimicrobial Resistance


 Please wait while we load your content...
Please wait while we load your content...