Rapid synthesis of carbonucleoside phophonate analogues as potential antiviral agents via a hydrophosphonylation reaction of ethynyl carbocyclic precursors†
Abstract
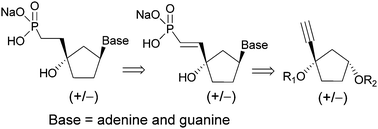
A racemic synthesis of new carbocyclic nucleoside ethyl or vinylphosphonate analogues bearing purine bases (adenine and guanine) was accomplished using racemic (+/−)-4-O-protected-2-cyclopentanone. All compounds were prepared using a hydrophosphonylation reaction of ethynyl carbocyclic precursors followed by a Mitsunobu coupling reaction between the heterocyclic bases. After deprotection, the compounds were evaluated for their activity against a large number of viruses. However, none of them showed significant antiviral activity or cytotoxicity.


 Please wait while we load your content...
Please wait while we load your content...