Complexation thermodynamics of tetraalkyl diglycolamides with trivalent f-elements in ionic liquids: spectroscopic, microcalorimetric and computational studies†
Abstract
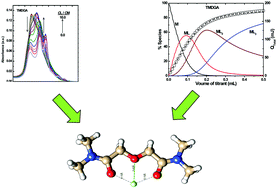
The thermodynamics of lanthanide complexation with a series of diglycolamides (DGAs) containing varying alkyl chain lengths (methyl, ethyl, n-butyl and n-hexyl) was studied in [C4mim][Tf2N], a room temperature ionic liquid. The stability constants, enthalpies and entropies of complexation were determined by spectrophotometry and calorimetry. All the DGA ligands formed a 1 : 3 (Nd3+/DGA) complex as the limiting species, and their stability constants increased linearly with increasing alkyl chain length. The stability constants of the Nd3+/DGA complexes are orders of magnitude higher in [C4mim][Tf2N] as compared to those observed in aqueous medium. For all the complexes, the enthalpy of complexation was negative with a positive entropy change, indicating that the complexation was driven by both enthalpy and entropy. The enthalpy changes observed in [C4mim][Tf2N] medium were more exothermic than those in the aqueous medium. Fluorescence lifetime data indicated that the complexation proceeded via the replacement of water molecules from the primary coordination sphere of the metal ion. DFT calculations were performed on the structures of the different Nd3+/tetramethyl DGA (TMDGA) complexes, and the corresponding free energies in both gas and solution phases were calculated. Insights into the structural features by DFT studies confirmed that the nature of Ln3+/DGA complexes formed in [C4mim][Tf2N], n-dodecane, and aqueous medium and that in the crystalline state is identical.


 Please wait while we load your content...
Please wait while we load your content...