The anion impact on the self-assembly of naphthalene diimide diimidazolium salts†
Abstract
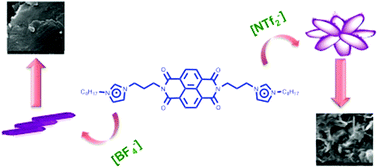
Naphthalene diimide diimidazolium salts differing in the anion nature were synthesized and their properties as well as their self-assembly behaviour were investigated. In particular, we took into consideration the N,N′-bis-(1-octyl-3-propylimidazolium)-naphthalene diimide cations and anions differing in size, shape and coordination abilities like [I−], [BF4−] and [NTf2−]. After determination of thermal behaviour, using differential scanning calorimetry and thermal gravimetric analysis, the electrochemical stability and redox properties were assessed using cyclic voltammetry. The self-assembly behaviour of the salts was investigated using concentration and temperature-dependent spectroscopic studies (UV-vis and fluorescence). Data collected demonstrate the ability of salts used to aggregate through an isodesmic pathway. Interestingly, the aggregation is featured by an enhanced emission event. The properties of aggregates were also investigated in the solid state using fluorescence measurements and scanning electron microscopy (SEM). On the whole, data collected show that the properties of salts and of aggregates that they are able to form are heavily dependent on the anion nature. Salts used combine the properties of a semiconductor, like the naphthalene diimide core, with the ones of imidazolium salts and could have interesting applications as materials for optoelectronic and conservation of energy.


 Please wait while we load your content...
Please wait while we load your content...