Polyethylene glycol (PEG-400) promoted as an efficient and recyclable reaction medium for the one-pot eco-friendly synthesis of functionalized isoxazole substituted spirooxindole derivatives†
Abstract
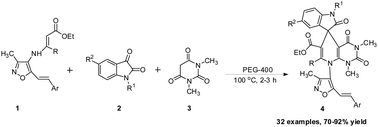
An efficient, inexpensive and environmentally friendly synthesis of novel isoxazole substituted spirooxindole-pyridopyrimidine/indenopyridine/chromenopyridine/naphthyridine/quinoline derivatives has been developed via one-pot three-component reaction of isoxazolyl enamino esters, isatins and 1,3-dimethylbarbituric acid/1,3-indandione/chromene-2,4-dione/quinoline-2,4-dione/naphthalene-1,2,4-trione/dimedone using PEG-400 as a solvent and catalyst. Twenty-six isoxazolyl enamino esters, seven 1,3-diketo compounds, and eleven substituted isatins were selected for the library validation. The advantages of this method are its environmentally safe nature, catalyst free nature, operational simplicity, metal free operation, diverse substrate scope, high yield, excellent functional group tolerance, and lower reaction time and PEG-400 can be recovered and reused.


 Please wait while we load your content...
Please wait while we load your content...