A new procedure to obtain ε-caprolactam catalyzed by a guanidinium salt†
Abstract
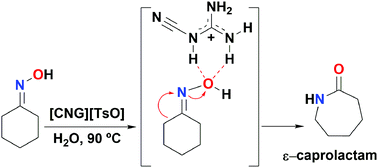
A new procedure to prepare ε-caprolactam by the Beckmann rearrangement of cyclohexanone oxime is described. Treatment of the oxime with the novel salt cyanoguanidinium tosylate affords ε-caprolactam without the need of any other promoter.


 Please wait while we load your content...
Please wait while we load your content...